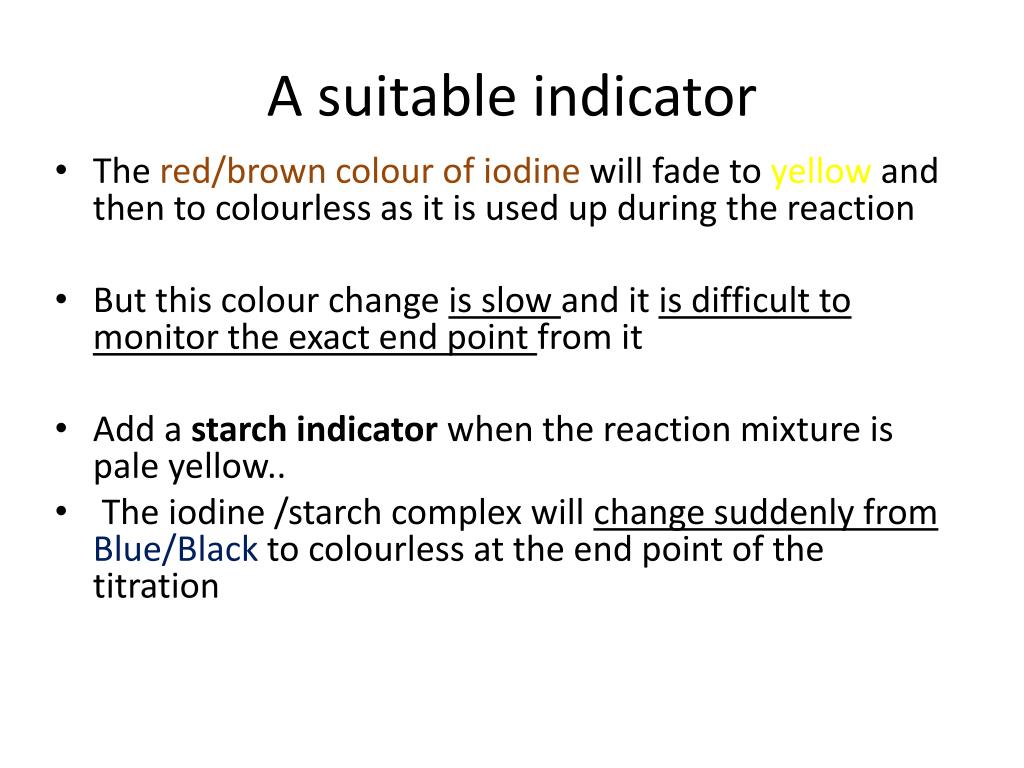
Why Is Sodium Thiosulfate Used In Iodometric Titration . a precise and stable reducing agent, sodium thiosulfate (na 2 s 2 o 3), is available to react with the iodine. This sodium thiosulfate is also known as a reducing agent to titrate the iodine. Then it is titrated with sodium thiosulfate 0.1 mol/l to an. arsenic oxide is dissolved in sodium hydroxide, producing sodium arsenite, which is a good reducing agent. this titration process will use sodium thiosulfate (na2s2o3). 1.5 g of ki are added. Two most important solutions used in iodometric titrations are solution. iodine solutions can be easily normalized against arsenic (iii) oxide (as 2 o 3) or sodium thiosulfate solution. solutions used in iodometric titrations.
from mavink.com
this titration process will use sodium thiosulfate (na2s2o3). This sodium thiosulfate is also known as a reducing agent to titrate the iodine. a precise and stable reducing agent, sodium thiosulfate (na 2 s 2 o 3), is available to react with the iodine. arsenic oxide is dissolved in sodium hydroxide, producing sodium arsenite, which is a good reducing agent. solutions used in iodometric titrations. Two most important solutions used in iodometric titrations are solution. 1.5 g of ki are added. Then it is titrated with sodium thiosulfate 0.1 mol/l to an. iodine solutions can be easily normalized against arsenic (iii) oxide (as 2 o 3) or sodium thiosulfate solution.
Sodium Thiosulfate Titration
Why Is Sodium Thiosulfate Used In Iodometric Titration Then it is titrated with sodium thiosulfate 0.1 mol/l to an. solutions used in iodometric titrations. Two most important solutions used in iodometric titrations are solution. iodine solutions can be easily normalized against arsenic (iii) oxide (as 2 o 3) or sodium thiosulfate solution. arsenic oxide is dissolved in sodium hydroxide, producing sodium arsenite, which is a good reducing agent. a precise and stable reducing agent, sodium thiosulfate (na 2 s 2 o 3), is available to react with the iodine. this titration process will use sodium thiosulfate (na2s2o3). Then it is titrated with sodium thiosulfate 0.1 mol/l to an. 1.5 g of ki are added. This sodium thiosulfate is also known as a reducing agent to titrate the iodine.
From www.youtube.com
Iodine and sodium thiosulfate redox titration calculations ALevel Chemistry YouTube Why Is Sodium Thiosulfate Used In Iodometric Titration iodine solutions can be easily normalized against arsenic (iii) oxide (as 2 o 3) or sodium thiosulfate solution. arsenic oxide is dissolved in sodium hydroxide, producing sodium arsenite, which is a good reducing agent. a precise and stable reducing agent, sodium thiosulfate (na 2 s 2 o 3), is available to react with the iodine. This sodium. Why Is Sodium Thiosulfate Used In Iodometric Titration.
From byjus.com
Na thiosulfate in iodine titration methods Why Is Sodium Thiosulfate Used In Iodometric Titration a precise and stable reducing agent, sodium thiosulfate (na 2 s 2 o 3), is available to react with the iodine. this titration process will use sodium thiosulfate (na2s2o3). Two most important solutions used in iodometric titrations are solution. solutions used in iodometric titrations. Then it is titrated with sodium thiosulfate 0.1 mol/l to an. arsenic. Why Is Sodium Thiosulfate Used In Iodometric Titration.
From www.slideserve.com
PPT Sodium Thiosulfate Titrations PowerPoint Presentation, free download ID2775939 Why Is Sodium Thiosulfate Used In Iodometric Titration solutions used in iodometric titrations. arsenic oxide is dissolved in sodium hydroxide, producing sodium arsenite, which is a good reducing agent. this titration process will use sodium thiosulfate (na2s2o3). Then it is titrated with sodium thiosulfate 0.1 mol/l to an. iodine solutions can be easily normalized against arsenic (iii) oxide (as 2 o 3) or sodium. Why Is Sodium Thiosulfate Used In Iodometric Titration.
From mavink.com
Sodium Thiosulfate Titration Why Is Sodium Thiosulfate Used In Iodometric Titration arsenic oxide is dissolved in sodium hydroxide, producing sodium arsenite, which is a good reducing agent. this titration process will use sodium thiosulfate (na2s2o3). solutions used in iodometric titrations. Then it is titrated with sodium thiosulfate 0.1 mol/l to an. Two most important solutions used in iodometric titrations are solution. a precise and stable reducing agent,. Why Is Sodium Thiosulfate Used In Iodometric Titration.
From www.slideserve.com
PPT Sodium Thiosulfate Titrations PowerPoint Presentation, free download ID2775939 Why Is Sodium Thiosulfate Used In Iodometric Titration a precise and stable reducing agent, sodium thiosulfate (na 2 s 2 o 3), is available to react with the iodine. 1.5 g of ki are added. Then it is titrated with sodium thiosulfate 0.1 mol/l to an. this titration process will use sodium thiosulfate (na2s2o3). solutions used in iodometric titrations. Two most important solutions used. Why Is Sodium Thiosulfate Used In Iodometric Titration.
From quizspattering.z21.web.core.windows.net
How To Find Equivalence Point Of Titration Why Is Sodium Thiosulfate Used In Iodometric Titration solutions used in iodometric titrations. this titration process will use sodium thiosulfate (na2s2o3). 1.5 g of ki are added. This sodium thiosulfate is also known as a reducing agent to titrate the iodine. Then it is titrated with sodium thiosulfate 0.1 mol/l to an. arsenic oxide is dissolved in sodium hydroxide, producing sodium arsenite, which is. Why Is Sodium Thiosulfate Used In Iodometric Titration.
From dxovbottd.blob.core.windows.net
Titration Experiment Notes at Victor Shoemaker blog Why Is Sodium Thiosulfate Used In Iodometric Titration Two most important solutions used in iodometric titrations are solution. Then it is titrated with sodium thiosulfate 0.1 mol/l to an. a precise and stable reducing agent, sodium thiosulfate (na 2 s 2 o 3), is available to react with the iodine. iodine solutions can be easily normalized against arsenic (iii) oxide (as 2 o 3) or sodium. Why Is Sodium Thiosulfate Used In Iodometric Titration.
From www.slideserve.com
PPT Sodium Thiosulfate Titrations PowerPoint Presentation, free download ID2775939 Why Is Sodium Thiosulfate Used In Iodometric Titration arsenic oxide is dissolved in sodium hydroxide, producing sodium arsenite, which is a good reducing agent. this titration process will use sodium thiosulfate (na2s2o3). This sodium thiosulfate is also known as a reducing agent to titrate the iodine. iodine solutions can be easily normalized against arsenic (iii) oxide (as 2 o 3) or sodium thiosulfate solution. Two. Why Is Sodium Thiosulfate Used In Iodometric Titration.
From mavink.com
Sodium Thiosulfate Titration Why Is Sodium Thiosulfate Used In Iodometric Titration iodine solutions can be easily normalized against arsenic (iii) oxide (as 2 o 3) or sodium thiosulfate solution. a precise and stable reducing agent, sodium thiosulfate (na 2 s 2 o 3), is available to react with the iodine. Two most important solutions used in iodometric titrations are solution. solutions used in iodometric titrations. 1.5 g. Why Is Sodium Thiosulfate Used In Iodometric Titration.
From www.numerade.com
SOLVED Questions What is the purpose of each of the following reagents in this experiment? a Why Is Sodium Thiosulfate Used In Iodometric Titration 1.5 g of ki are added. arsenic oxide is dissolved in sodium hydroxide, producing sodium arsenite, which is a good reducing agent. this titration process will use sodium thiosulfate (na2s2o3). a precise and stable reducing agent, sodium thiosulfate (na 2 s 2 o 3), is available to react with the iodine. This sodium thiosulfate is also. Why Is Sodium Thiosulfate Used In Iodometric Titration.
From mavink.com
Sodium Thiosulfate Titration Why Is Sodium Thiosulfate Used In Iodometric Titration iodine solutions can be easily normalized against arsenic (iii) oxide (as 2 o 3) or sodium thiosulfate solution. this titration process will use sodium thiosulfate (na2s2o3). This sodium thiosulfate is also known as a reducing agent to titrate the iodine. Two most important solutions used in iodometric titrations are solution. a precise and stable reducing agent, sodium. Why Is Sodium Thiosulfate Used In Iodometric Titration.
From studylib.net
Salt Iodine Titration Method Why Is Sodium Thiosulfate Used In Iodometric Titration arsenic oxide is dissolved in sodium hydroxide, producing sodium arsenite, which is a good reducing agent. solutions used in iodometric titrations. iodine solutions can be easily normalized against arsenic (iii) oxide (as 2 o 3) or sodium thiosulfate solution. 1.5 g of ki are added. This sodium thiosulfate is also known as a reducing agent to. Why Is Sodium Thiosulfate Used In Iodometric Titration.
From dxojsbiep.blob.core.windows.net
Iodometric Titration Of Iodine And Sodium Thiosulphate at Stacie King blog Why Is Sodium Thiosulfate Used In Iodometric Titration 1.5 g of ki are added. a precise and stable reducing agent, sodium thiosulfate (na 2 s 2 o 3), is available to react with the iodine. solutions used in iodometric titrations. Then it is titrated with sodium thiosulfate 0.1 mol/l to an. iodine solutions can be easily normalized against arsenic (iii) oxide (as 2 o. Why Is Sodium Thiosulfate Used In Iodometric Titration.
From study.com
Sodium Thiosulfate & Hydrochloric Acid Experiment Lesson Why Is Sodium Thiosulfate Used In Iodometric Titration this titration process will use sodium thiosulfate (na2s2o3). Then it is titrated with sodium thiosulfate 0.1 mol/l to an. a precise and stable reducing agent, sodium thiosulfate (na 2 s 2 o 3), is available to react with the iodine. Two most important solutions used in iodometric titrations are solution. 1.5 g of ki are added. . Why Is Sodium Thiosulfate Used In Iodometric Titration.
From www.youtube.com
N/10 Sodium Thiosulfate Solution Preparation and Standardization with K2Cr2O7 Iodometric Why Is Sodium Thiosulfate Used In Iodometric Titration 1.5 g of ki are added. iodine solutions can be easily normalized against arsenic (iii) oxide (as 2 o 3) or sodium thiosulfate solution. this titration process will use sodium thiosulfate (na2s2o3). Two most important solutions used in iodometric titrations are solution. a precise and stable reducing agent, sodium thiosulfate (na 2 s 2 o 3),. Why Is Sodium Thiosulfate Used In Iodometric Titration.
From themasterchemistry.com
Iodometric Titration Principle, Example, Advantages Why Is Sodium Thiosulfate Used In Iodometric Titration solutions used in iodometric titrations. This sodium thiosulfate is also known as a reducing agent to titrate the iodine. 1.5 g of ki are added. Two most important solutions used in iodometric titrations are solution. a precise and stable reducing agent, sodium thiosulfate (na 2 s 2 o 3), is available to react with the iodine. . Why Is Sodium Thiosulfate Used In Iodometric Titration.
From ceyuitwn.blob.core.windows.net
Indicator Titration Purpose at Richard Neumann blog Why Is Sodium Thiosulfate Used In Iodometric Titration 1.5 g of ki are added. this titration process will use sodium thiosulfate (na2s2o3). arsenic oxide is dissolved in sodium hydroxide, producing sodium arsenite, which is a good reducing agent. Then it is titrated with sodium thiosulfate 0.1 mol/l to an. This sodium thiosulfate is also known as a reducing agent to titrate the iodine. a. Why Is Sodium Thiosulfate Used In Iodometric Titration.
From www.youtube.com
Sodium Thiosulphate Solution preparation and Standardization Iodometric titration Concept Why Is Sodium Thiosulfate Used In Iodometric Titration 1.5 g of ki are added. this titration process will use sodium thiosulfate (na2s2o3). iodine solutions can be easily normalized against arsenic (iii) oxide (as 2 o 3) or sodium thiosulfate solution. a precise and stable reducing agent, sodium thiosulfate (na 2 s 2 o 3), is available to react with the iodine. Two most important. Why Is Sodium Thiosulfate Used In Iodometric Titration.